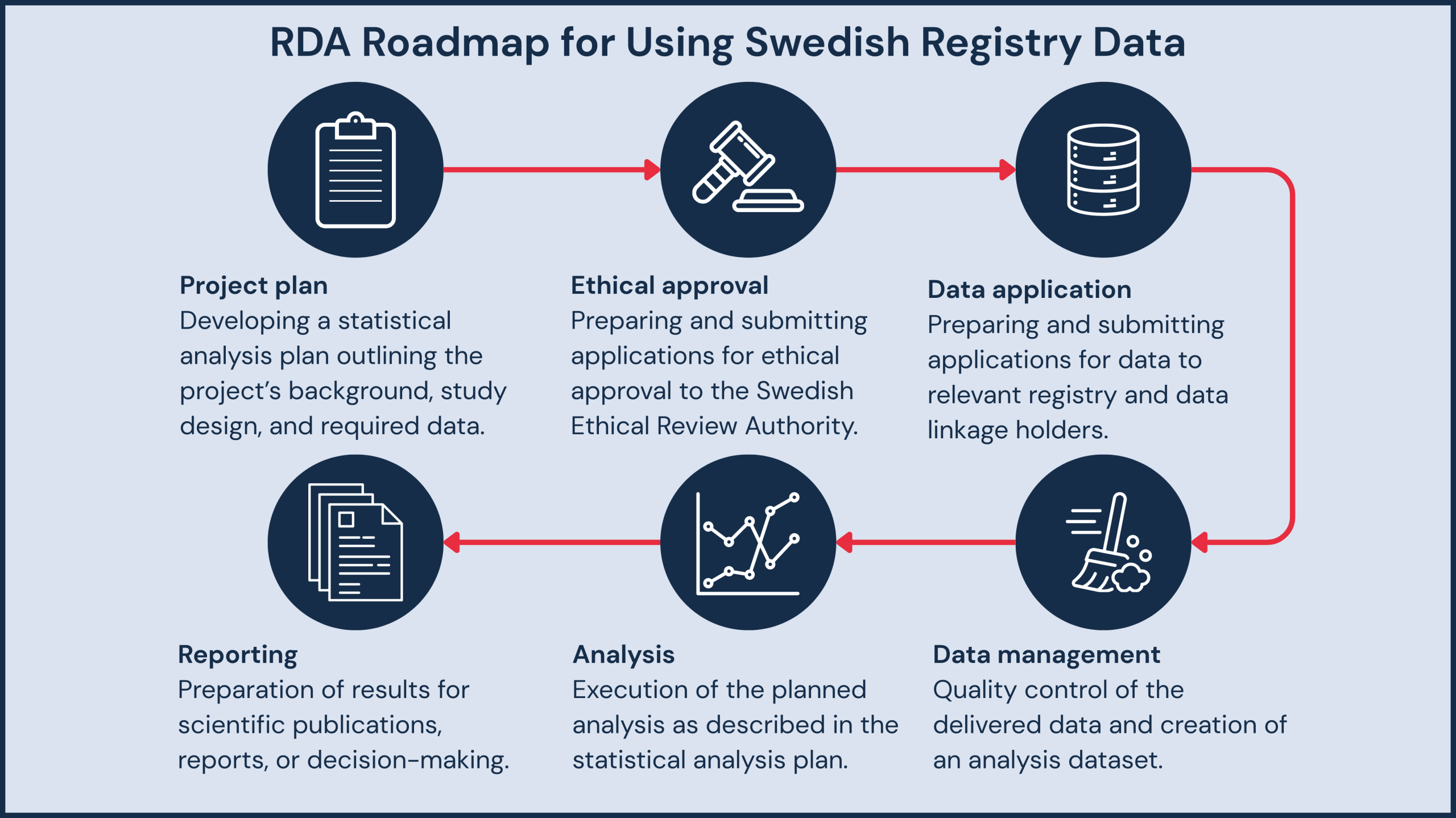
Why Swedish Registers?
Sweden offers researchers a unique advantage: comprehensive national registries with population-level data spanning decades. The register infrastructure includes both medical registers (capturing healthcare utilization, diagnoses, treatments, and outcomes—some dating back over 60 years) and sociodemographic registers (covering demographics, education, employment, and social factors). All registers are linkable at the individual level using the Swedish national ID number, enabling comprehensive analyses across Sweden’s population of 10 million. This rich data infrastructure makes Sweden an exceptional setting for robust, real-world research in clinical, epidemiological, and health economic applications.
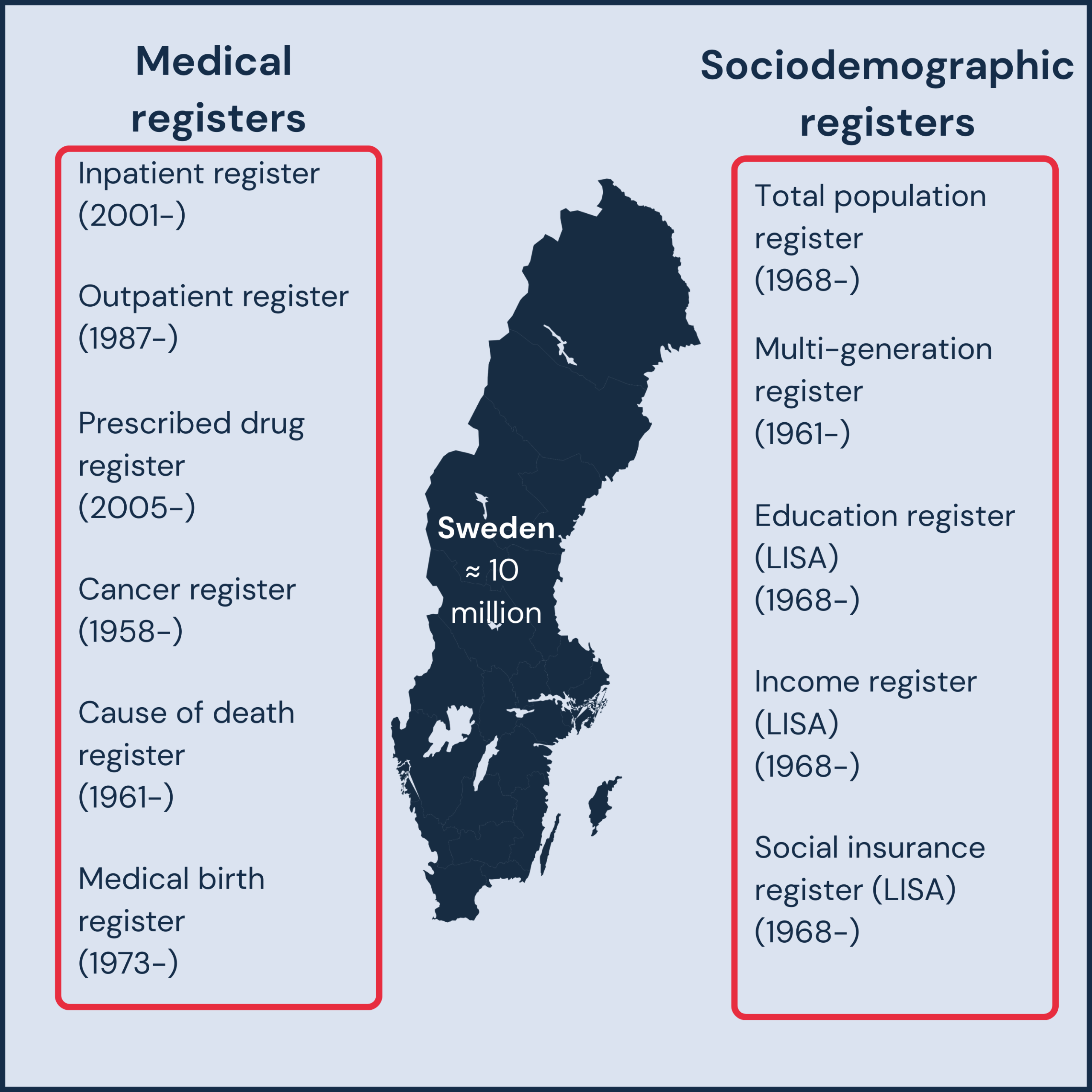
Besides the national registers, Sweden has around 150 disease-specific registers which include information on diagnostic variables, genetic traits, treatments, and follow-up data. Using the Swedish national ID number each register can be linked to all national registers or other quality registers. To assure a high quality and coverage each quality register is annually reviewed by the Swedish national authorities and assigned a certified quality level ranging from three (lowest) to one (highest). Level one quality registers are required to have a coverage of at least 85%.